CIT-013 Development
Drug development of CIT-013 Preclinical data
Citryll develops a first in class therapeutic antibody that targets Neutrophil Extracellular Traps (NET) formation for the treatment of a range of human diseases with potential for SLE, RA, vasculitis, IPF and other indications.
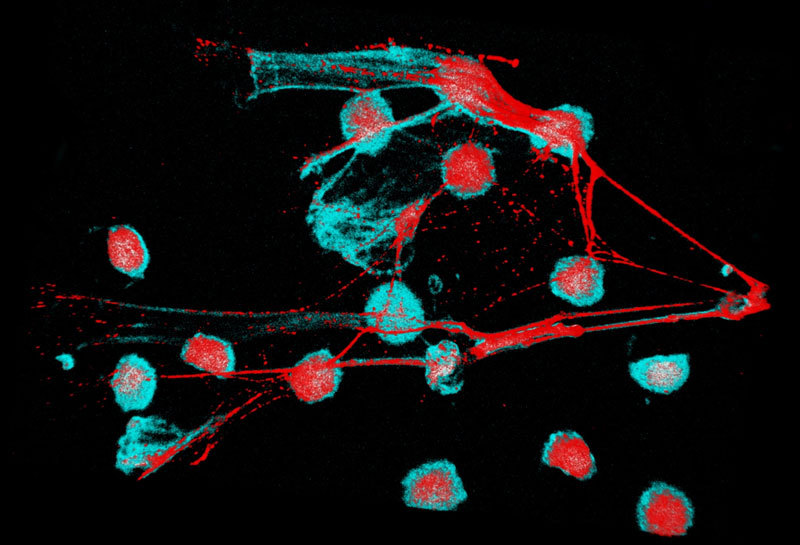
The initial therapeutic antibodies, designated therapeutic Anti- Citrullinated Protein Antibodies or tACPAs, and prior to the optimisation of CIT-013, were identified among ACPAs cloned from RA patient B lymphocytes. We have since demonstrated tACPA’s cellular MoA to be interfering with NET biology, using primary human neutrophils from healthy donors as well as RA and SLE patients and numerous in vivo pharmacology studies in disease animal models.
- Therapeutic use of tACPAs in an RA mouse model resulted in the arrest of inflammation and prevents a further increase of the inflammatory response, and significantly decreased joint damage, close to normal (Chirivi et al., J Clin Cell Immunol 2013, S6, 1-13) and resulted in near disappearance of NET is in inflamed joints (Chirivi et al, 2020).
- Use in 2 different mouse RA models (CAIA and CIA) significantly prevented the onset of inflammation. Histological analysis of inflamed joints treated mice revealed a significant decrease in neutrophil influx and joint damage, as compared to control animals to near normal tissue. tACPA also strongly inhibits the formation of NETs in the affected joints of these mice.
- The tACPA development candidate CIT-013 recognises the citrullinated N-terminus of histone-2A and histone-4 which are present in human NETs and are essential for NET formation. Neutrophils and aberrant NET formation contribute to the induction and propagation of inflammation. NETs are believed to be central for developing autoimmunity in human RA, SLE and other indications and are present in RA joints. NETs also play a central role in sepsis induced organ failure. NETs are found in large numbers of lungs of chronically infected lungs of Cystic fibrosis, lungs of COPD patients and severe asthma, virally induced exacerbation of Asthma, inhibiting lung function and recently have been shown to be important in acute respiratory distress syndrome (ARDS) in COVID19.
- tACPAs are active in preclinical models for Idiopathic Pulmonary Fibrosis (IPF) and colitis. In the IPF animal model we find that tACPA protects against tissue damage in the lung. Furthermore, strongly decreased circulating neutrophil levels were observed in tACPA treated mice compared to mice that received a control antibody in both animal models and we have demonstrated in vivo inhibition of NET formation in a pristine mouse model.
Development candidate CIT-013
Near human lead antibodies were generated with appropriate potencies and favourable characteristics for development including low aggregation, good stability and good production in CHO based stable cell lines. The development candidate CIT-013 was selected from a set of optimised antibodies. The production of CIT-013 drug substance and drug product has been
conducted by our CMC partner Lonza including cell line generation, cloning, bioreactor scaling, process and formulation development, technical batch and 1000 liter GMP lot production. We started clinical studies in healthy volunteers in August 2021 and plan to start patient studies in 2023.
In summary, the current status is: preclinical proof of concept using SLE and RA patient neutrophils, 2 animal models for RA, early data in IPF and colitis, sepsis model and pristane induced NET formation. We are currently expanding preclinical data set by testing in additional therapeutic models.
NETosis inhibition is expected to decrease auto-antigen release with the potential to disrupt the autoimmunity cycle and NET mediated tissue damage due to release of histone and other toxic NET components. Moreover it promotes the clearance of preexisting pro-inflammatory NETs by promoting macrophage mediated digestion of CIT-013 NETs and preNETs and counters histone toxicity.
Clinical development towards PoC of CIT-013 a first in class antagonist of NETosis biology
CIT-013 has a number of life cycle opportunities and after careful analysis and feasibility studies we have chosen two indications for which Citryll intends to obtain clinical Proof of Concept that CIT-013 can inhibit NET formation and promote NET clearance through NET and disease biomarkers as well as exploratory clinical efficacy readouts.
First in human studies (FIH) include a confirmatory SAD arm, a PK/PD study after nano-dosing with LPS that induces NETosis, a subcutaneous dosing arm and finally a safety/PK study in RA patient volunteers. The first three parts of the FIH studies are near finalisation at the time of writing in Q4 2022.The first in human studies will be followed by phase 2a clinical PoC studies in Rheumatoid arthritis and Hidradenitis suppurativa patients. A third phase 2a PoC study in a respiratory disease is being considered.
